Cultivating Cerebral Organoids
This is intended as a guide only; for full experimental details please read the reference provided.
In Brief Download the PDF of this protocol
Lancaster et al. describe a protocol to generate cerebral organoids from H9 ES cells.
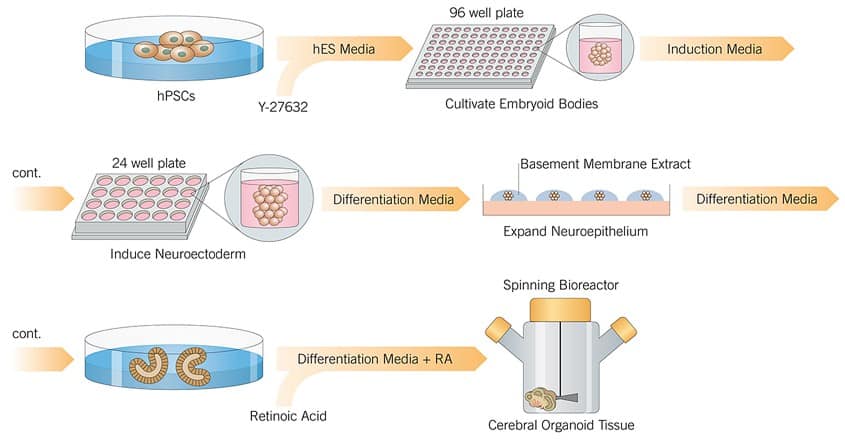
On Day 0 ES cells or iPSCs were dissociated from mouse embryonic stem cells (MEFs) and separated to make single cells. Cells were then plated in 96-well plates in human ES media containing 50 μM Y-27632. Embryoid bodies were fed every other day for 6 days.
On Day 6 cells are transferred to 24 well plates and grown in induction media. They are fed every other day for 5 further days.
On Day 11 tissue is transferred to droplet Matrigels containing differentiation media. After 4 days of stationary growth the tissue droplets are moved to a spinning bioreactor, and grown in differentiation media containing retinoic acid (RA).
Cocktails
| hES Media | Neural Induction Media | Differentiation Media | Differentiation Media + RA | ||||
|---|---|---|---|---|---|---|---|
| FGF (233-FB) | 4 ng ml-1 | DMEM:F12 medium | DMEM:F12 medium and neurobasal containing N2 supplement (1:200) | Same as differentiation + | |||
| Y-27632 (Cat.No. 1254) | 50 μM | N2 Supplement | 1:100 | B 27 supplement w/o vitamin A (equivalent to N21-MAX Vitamin A Free (AR009)) | 1:100 | B 27 supplement with vitamin A (equivalent to N21-MAX with Vitamin A (AR008)) | 1:100 |
| Glutamax (equivalent to GlutaminePlus (B90210) | 2 Mercapotoethanol | 3.5 μl/L | Retinoic Acid (Cat.No. 0695) | ||||
| MEM-NEAA | Insulin (Cat.No. 3435) | 1:4000 | |||||
| Heparin sodium salt (Cat.No. 2812) | 1 mg/ml | Glutamax | 1:100 | ||||
| MEM-NEAA | |||||||