Hippo and YAP/TAZ Signaling
The Hippo signaling pathway regulates cell proliferation and differentiation, playing a key role in the regulation of tissue development and organ size, through regulation of TEF transcription factors. Dysregulation is implicated in the development of multiple cancers.
Hippo and YAP/TAZ Signaling Inhibitors |
|
|---|---|
| Cat. No. | Product Name / Activity |
| 4892 | (R)-PFI 2 hydrochloride |
| Inhibits YAP nuclear translocation | |
| 7832 | TAT-PDHPS1 |
| YAP inhibitor | |
| 7917 | TM2 TEAD inhibitor |
| Potent and reversible inhibitor of TEAD mediated Hippo/Yap signalling | |
| 5305 | Verteporfin |
| YAP inhibitor | |
| 6482 | XMU MP 1 |
| Potent and selective MST1/2 inhibitor; enhances downstream activity of YAP; orally bioavailable | |
Hippo and YAP/TAZ Signaling Activators |
|
| Cat. No. | Product Name / Activity |
| 3603 | Kaempferol |
| TAZ activator; promotes osteogenesis from mesenchymal stem cells; also activates mitochondrial Ca2+ uniporter | |
| 8175 | NIBR LTSi |
| YAP signaling activator; LATS kinase inhibitor | |
Other |
|
| Cat. No. | Product Name / Activity |
| 2272 | Ro 08-2750 |
| Regulates YAP oncogenic activity; also inhibits NGF receptor tyrosine kinase | |
The Hippo signaling pathway regulates cell proliferation and differentiation, playing a key role in the regulation of tissue development and organ size through restriction of proliferation and promotion of apoptosis. First identified in Drosophila melanogaster, this pathway is conserved in mammals and regulates transcription of a wide range of genes through the interaction of the Yes associated protein (YAP)/Transcriptional coactivator with PDZ binding domain (TAZ) complex with transciptional enhancer factor (TEF) family members.
The Hippo/YAP/TAZ pathway is not activated by a single ligand, but rather integrates with multiple other intracellular pathways (including GPCR, TGFβ, EGFR and NOTCH signaling) to confer their downstream functions. Additionally the Hippo signaling pathway regulates the Wnt signaling pathway. Activation of the kinase module, consisting of MST1/2 (mammal homologs of Drosophila protein, Hippo) and LATS1/2 (mammal homologs of Drosophila protein, Warts) with activating proteins, causes MST1/2 to phosphorylate LATS1/2. LATS1/2 then phosphorylates YAP/TAZ leading to deactivation, cytoplasmic retention and priming of YAP/TAZ for proteasomal degradation. With no phosphorylation, YAP/TAZ remains active, binding to TEF transcription factors to induce expression of genes involved in cell proliferation, survival and migration.
The Hippo/YAP/TAZ signaling pathway
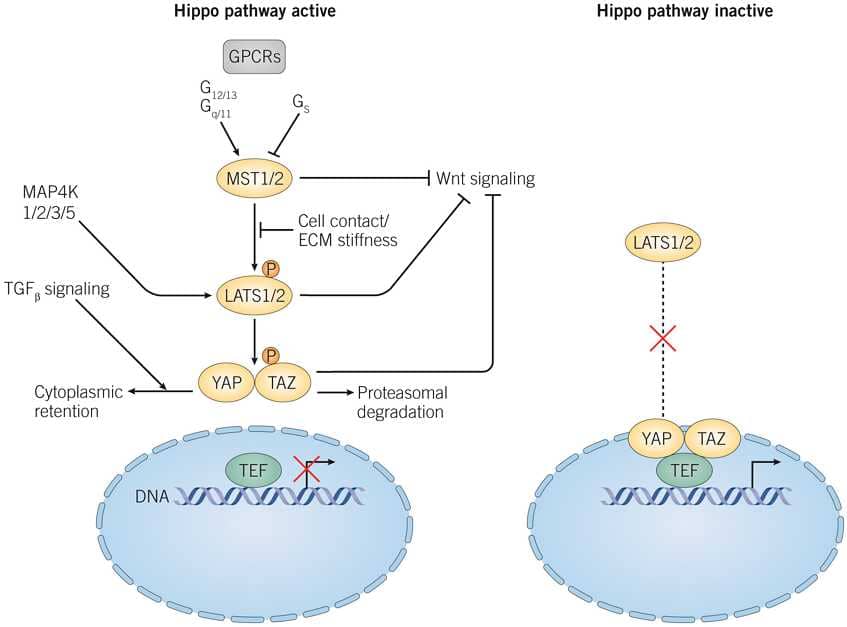
Figure 1: The Hippo/YAP/TAZ signaling pathway. ECM, Extracellular matrix; GPCRs, G-Protein coupled receptors; TAZ, Transcriptional coactivator with PDZ binding domain; TEF, Transciptional enhancer factor; TGFβ, Transforming growth factor; YAP, Yes associated protein.
Adapted from Hansen et al (2015) Trends Cell Biol. 25 499.
The Hippo pathway is modulated by both physical and soluble factors. Cell-cell contact at high cell densities, with associated increased numbers of tight and adherens junctions and high extracellular matrix stiffness leads to LATS kinase inhibition. Contact inhibition of the YAP/TAZ-TEF interaction is critical for embryonic development. Soluble factors acting through GPCR signaling will activate or inhibit the Hippo pathway dependent on Gα protein subtype. Additionally, the metabolic status of the cell and position in the cell cycle can modulate this pathway.
Dysregulation of the Hippo pathway has been implicated in multiple cancers, with genetic inactivation of the pathway resulting in overgrowth of a range of organs in Drosophila and rodent models. Inactivation of YAP/TAZ impairs wound healing while activation of the Hippo pathway shows therapeutic potential in regenerative medicine.
Literature for Hippo and YAP/TAZ Signaling
Tocris offers the following scientific literature for Hippo and YAP/TAZ Signaling to showcase our products. We invite you to request* your copy today!
*Please note that Tocris will only send literature to established scientific business / institute addresses.
Stem Cells Scientific Review
Written by Kirsty E. Clarke, Victoria B. Christie, Andy Whiting and Stefan A. Przyborski, this review provides an overview of the use of small molecules in the control of stem cell growth and differentiation. Key signaling pathways are highlighted, and the regulation of ES cell self-renewal and somatic cell reprogramming is discussed. Compounds available from Tocris are listed.
Cell Cycle & DNA Damage Repair Poster
In normal cells, each stage of the cell cycle is tightly regulated, however in cancer cells many genes and proteins that are involved in the regulation of the cell cycle are mutated or over expressed. This poster summarizes the stages of the cell cycle and DNA repair. It also highlights strategies for enhancing replicative stress in cancer cells to force mitotic catastrophe and cell death.





