Submit a Review & Earn an Amazon Gift Card
You can now submit reviews for your favorite Tocris products. Your review will help other researchers decide on the best products for their research. Why not submit a review today?!
Submit ReviewStaurosporine is a broad spectrum protein kinase inhibitor. Enzymes inhibited include protein kinase C, p60v-src tyrosine protein kinase, protein kinase A, and CaM kinase II (IC50 values are 3nM, 6 nM, 7 nM and 20 nM, respectively). Staurosporine reduces nuclear myosin heavy chain 9 phosphorylation which inhibits gastric cancer cell progression in transgenic mouse models. Staurosporine inhibits cell viability and promotes apoptosis in oral and pancreatic cancer cells. Staurosporine also enhances efficiency of lentiviral transduction of human hematopoietic stem and progenitor cells by 2-fold, induces dopaminergic axonal outgrowth in vitro and triggers mitophagy. Staurosporine has high affinity (Kd = 100 nM) for the yjdF aptamer. The riboswitch function of yjdF motif RNAs is activated by Staurosporine and leads to robust reporter gene expressions in B. subtilis.
Staurosporine synthesized to Ancillary Material Grade is also available.
 View Larger
View Larger
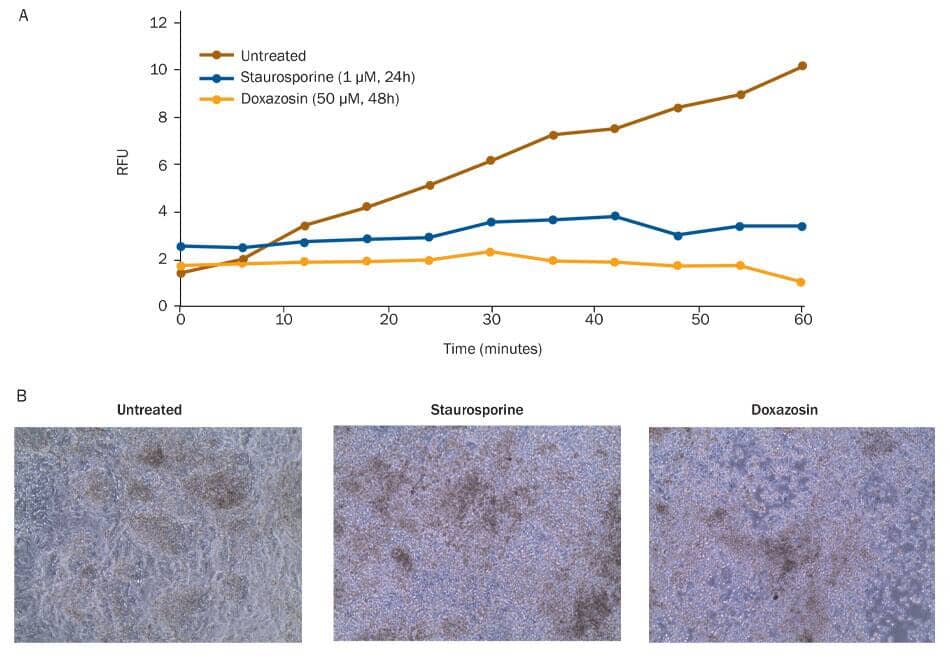
Reduced viability of differentiated cardiomyocytes exposed to cardiotoxic small molecules. The viability of differentiated cardiomyocytes was assessed using a Resazurin metabolism assay. A) Untreated cells metabolize Resazurin (Catalog # AR002) to produce resorufin, a fluorescent molecule that can be measured using a fluorometric plate reader. Resorufin fluorescence accumulated in untreated cardiomyocytes. Cells treated with the cardiotoxic small molecules Staurosporine (blue; Catalog # 1285), a non-selective protein kinase inhibitor, or Doxazosin (tan; Catalog # 2964), an alpha 1 antagonist, did not metabolize Resazurin, as shown by lack of fluorescence accumulation, indicating a loss of viability upon treatment. B) Cell morphology of untreated, Staurosporine-treated, and Doxazosin-treated cardiomyocytes was assessed by brightfield microscopy.
| M. Wt | 466.54 |
| Formula | C28H26N4O3 |
| Storage | Store at -20°C |
| Purity | ≥98% (HPLC) |
| CAS Number | 62996-74-1 |
| PubChem ID | 44259 |
| InChI Key | HKSZLNNOFSGOKW-FYTWVXJKSA-N |
| Smiles | O=C(NC6)C1=C6C4=C3C2=C1C8=C(C=CC=C8)N2[C@](C[C@@H](NC)[C@H]7OC)([H])O[C@]7(C)N3C5=CC=CC=C45 |
The technical data provided above is for guidance only. For batch specific data refer to the Certificate of Analysis.
Tocris products are intended for laboratory research use only, unless stated otherwise.
| Solvent | Max Conc. mg/mL | Max Conc. mM | |
|---|---|---|---|
| Solubility | |||
| DMSO | 23.33 | 50 |
The following data is based on the product molecular weight 466.54. Batch specific molecular weights may vary from batch to batch due to the degree of hydration, which will affect the solvent volumes required to prepare stock solutions.
| Concentration / Solvent Volume / Mass | 1 mg | 5 mg | 10 mg |
|---|---|---|---|
| 0.5 mM | 4.29 mL | 21.43 mL | 42.87 mL |
| 2.5 mM | 0.86 mL | 4.29 mL | 8.57 mL |
| 5 mM | 0.43 mL | 2.14 mL | 4.29 mL |
| 25 mM | 0.09 mL | 0.43 mL | 0.86 mL |
References are publications that support the biological activity of the product.
Ruegg and Burgess (1989) Staurosporine, K-252 and UCN-01: potent but nonspecific inhibitors of protein kinases. TiPS 10 218 PMID: 2672462
Tamaoki et al (1986) Staurosporine, a potent inhibitor of phospholipid/Ca2+ dependent protein kinase. Biochem.Biophys.Res.Commun. 135 397 PMID: 3457562
Yanagihara et al (1991) Staurosporine: an effective inhibitor for Ca2+/calmodulin-dependent protein kinase II. J.Neurochem. 56 294 PMID: 1846174
Lewis et al (2018) Staurosporine increases lentiviral vector transduction efficiency of human hematopoietic stem and progenitor cells. Mol.Ther.Methods Clin.Dev. 9 313 PMID: 30038935
Ye et al (2020) Nuclear MYH9-induced CTNNB1 transcription, targeted by staurosporin, promotes gastric cancer cell anoikis resistance and metastasis. Theranostics 10 7545 PMID: 32685004
Zhang et al (2005) Effect of protein kinase C alpha, caspase-3, and survivin on apoptosis of oral cancer cells induced by staurosporine. Acta Pharmacol.Sinica 26 1365 PMID: 16225760
Malsy et al (2019) Staurosporine induces apoptosis in pancreatic carcinoma cells PaTu 8988t and Panc-1 via the intrinsic signaling pathway. Eur.J.Med.Res. 24 5 PMID: 30686270
Wakita et al (2014) Staurosporine induces dopaminergic neurite outgrowth through AMP-activated protein kinase/mammalian target of rapamycin signaling pathway. Neuropharmacology 77 39 PMID: 24067927
Li et al (2016) The yjdF riboswitch candidate regulates gene expression by binding diverse azaaromatic compounds. RNA 22 530 PMID: 26843526
If you know of a relevant reference for Staurosporine, please let us know.
Keywords: Staurosporine, Staurosporine supplier, Non-Selective, protein, kinases, inhibitors, inhibits, Broad, Spectrum, viral, transduction, enhancers, enhances, antibiotic, AM-2282, STS, parkin, PINK, parkinsons, mitochondria, mitochondrial, apoptosis, high, affinity, to, yjdF, aptamer, activates, riboswitch, RNA, Protein, Kinase, Inhibitors, Viral, Transduction, Enhancers, DNA,, and, Synthesis, Organoids, 1285, Tocris Bioscience
Citations are publications that use Tocris products. Selected citations for Staurosporine include:
Hayes et al (2016) Integrative genomic and functional analysis of human oral squamous cell carcinoma cell lines reveals synergistic effects of FAT1 and CASP8 inactivation. Cancer Lett 383 106 PMID: 27693639
Dobson et al (2015) Caffeine Modulates Vesicle Release and Recovery at Cerebellar Parallel Fibre Terminals, Independently of Calcium and Cyclic AMP Signalling. Eur J Neurosci 10 e0125974 PMID: 25933382
Sonamoto et al (2015) Identification of a DYRK1A Inhibitor that Induces Degradation of the Target Kinase using Co-chaperone CDC37 fused with Luciferase nanoKAZ. Eneuro 5 12728 PMID: 26234946
Faulkner et al (2015) FMRP regulates neurogenesis in vivo in Xenopus laevis tadpoles. PLoS One 2 e0055 PMID: 25844398
Kawamoto et al (2012) Effect of activation of canonical Wnt signaling by the Wnt-3a protein on the susceptibility of PC12 cells to oxidative and apoptotic insults. PLoS One 45 58 PMID: 22124704
Kondo et al (2010) Poly(ADP-ribose) polymerase (PARP)-1-independent apoptosis-inducing factor (AIF) release and cell death are induced by eleostearic acid and blocked by α-tocopherol and MEK inhibition. Braz J Med Biol Res 285 13079 PMID: 20177052
Chen et al (2016) Androgen-Sensitized Apoptosis of HPr-1AR Human Prostate Epithelial Cells. Mol Pharmacol 11 e0156145 PMID: 27203692
Piccart et al (2015) Neurotensin Induces Presynaptic Depression of D2 DA Autoreceptor-Mediated Neurotransmission in Midbrain DArgic Neurons. J Neurosci 35 11144 PMID: 26245975
Barcomb et al (2013) Enzymatic activity of CaMKII is not required for its interaction with the glutamate receptor subunit GluN2B. J Biol Chem 84 834 PMID: 24056996
Mayer et al (2017) The microanatomic segregation of selection by apoptosis in the germinal center. Science 358 PMID: 28935768
Valvezan et al (2017) mTORC1 Couples Nucleotide Synthesis to Nucleotide Demand Resulting in a Targetable Metabolic Vulnerability. Cancer Cell 32 624 PMID: 29056426
Hejna et al (2017) High accuracy label-free classification of single-cell kinetic states from holographic cytometry of human melanoma cells. Sci Rep 7 11943 PMID: 28931937
Schönbrunn et al (2013) Development of highly potent and selective diaminothiazole inhibitors of cyclin-dependent kinases. J Med Chem 56 3768 PMID: 23600925
Valero et al (2011) Contractile effect of tachykinins on rabbit small intestine. Acta Pharmacol Sin 32 487 PMID: 21441943
O'Donnell et al (2015) IF. gamma induces protective non-canonical signaling pathways in primary neurons. J Neurochem 135 309 PMID: 26190522
Xu et al (2017) Matrine induces RIP3-dependent necroptosis in cholangiocarcinoma cells. Cell Death Discov 3 16096 PMID: 28179994
Park et al (2016) Pyrin inflammasome activation and RhoA signaling in the autoinflammatory diseases FMF and HIDS. Nat Immunol 17 914 PMID: 27270401
Valentine et al (2014) Small molecule screen yields inhibitors of Pseudomonas homoserine lactone-induced host responses. Cell Microbiol 16 1 PMID: 23910799
Jackson et al (2013) Pharmacological inhibition of pleckstrin homology domain leucine-rich repeat protein phosphatase is neuroprotective: differential effects on astrocytes. J Pharmacol Exp Ther 347 516 PMID: 24023368
Chen et al (2006) N-myc down-regulated gene 1 modulates the response of term human trophoblasts to hypoxic injury. J Biol Chem 281 2764 PMID: 16314423
Do you know of a great paper that uses Staurosporine from Tocris? Please let us know.
Average Rating: 4 (Based on 1 Review.)
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:
Used in cytotox control, 7CC. No issues at all, vial could be smaller to spin down easier